Associação Portuguesa de Investigação em Cancro
O fator de transcrição brachyury é um novo supressor tumoral em gliomas
O fator de transcrição brachyury é um novo supressor tumoral em gliomas
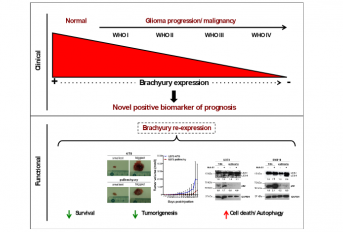
O trabalho recentemente publicado na revista ‘Journal of Pathology’ foi realizado no ICVS/3B’s, Escola de Medicina, Braga, e teve a parceria de Instituições nacionais e internacionais, incluindo o Hospital de Câncer de Barretos (São Paulo, Brasil) e The Institute of Cancer Research - ICR (Sutton, UK). O brachyury, importante factor de transcrição no desenvolvimento embrionário tem sido descrito como oncogene em cordomas e vários outros tumores sólidos. No entanto, o presente estudo sugere um papel oposto – de gene supressor tumoral - em gliomas. Neste estudo explorámos o papel clínico e funcional de brachyury. A expressão e importância clínica de brachyury foi estudada em mais de 2000 pacientes diagnosticados com gliomas, utilizando técnicas de imunohistoquímica, genómica e bioinformática. Verificámos que a expressão desta molécula está diminuída ou ausente em gliomas quando comparadas com tecidos normais. Adicionalmente, a perda de expressão de brachyury está correlacionada com gliomas mais agressivos (glioblastomas- grau IV de malignidade) e pior prognóstico. O papel de brachyury na gliomogénese foi explorado através de múltiplas abordagens in vitro e in vivo, e verificámos que a presença de brachyury induz uma regressão tumoral via modulação da autofagia celular.
Em sumário, neste trabalho demostramos que o papel de brachyury na tumorigénese pode ser dependente do tipo de tecido tumoral, e que atua como um supressor tumoral em gliomas, podendo constituir um novo biomarcador de prognóstico para estes tumores.
Autores e Afiliações:
Pinto F1,2,3,4, Costa ÂM1,2,3, Santos GC5, Matsushita MM5, Costa S1,2, Silva VAO6, Miranda-Gonçalves V1,2, Lopes CM7,8, Clara CA9, Becker AP6, Neder L10, Hajj GNM11, da Cunha IW12, Jones C13, Andrade RP1,2,14,15, Reis RM1,2,6.
1 Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal.
2 ICVS/3B's - PT Government Associate Laboratory, Braga/Guimarães, Portugal.
3 i3S - Instituto de Investigação e Inovação em Saúde, University of Porto, Portugal.
4 Institute of Molecular Pathology and Immunology of the University of Porto - IPATIMUP, Porto, Portugal.
5 Department of Pathology, Barretos Cancer Hospital Barretos, S. Paulo, Brazil.
6 Molecular Oncology Research Center, Barretos Cancer Hospital, S. Paulo, Brazil.
7 Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal.
8 Faculty of Pharmacy, University of Coimbra, Coimbra, Portugal.
9 Neurosurgery Department, Barretos Cancer Hospital, Barretos, S. Paulo, Brazil.
10 Department of Pathology and Forensic Medicine (APB, LN), Faculty of Medicine of Ribeirão Preto, University of São Paulo (FMRP-USP), São Paulo, Brazil.
11 International Research Center, AC Camargo Cancer Center, São Paulo, Brazil.
12 Department of Molecular Diagnosis, Anatomic Pathology Department, AC Camargo Cancer Center, São Paulo, Brazil.
13 Divisions of Molecular Pathology and Cancer Therapeutics, The Institute of Cancer Research (ICR), Sutton, UK.
14 Regenerative Medicine Program; Department of Medicine and Biomedical Sciences, University of Algarve, Faro, Portugal.
15 CBMR, Centre for Biomedical Research, Universidade do Algarve, Faro, Portugal.
Abstract:
The oncogene brachyury (TBXT) is a T-box transcription factor that is overexpressed in multiple solid tumors and is associated with tumor aggressiveness and poor patient prognosis. Gliomas comprise the most common and aggressive group of brain tumors, and at the present time the functional and clinical impact of brachyury expression has not previously been investigated in these neoplasms. Brachyury expression (mRNA and protein) was assessed in normal brain (n = 67), glioma tissues (n = 716) and cell lines (n = 42), and further in silico studies were undertaken using genomic databases totaling 3115 samples. Our glioma samples were analyzed for copy number (n = 372), promoter methylation status (n = 170), and mutation status (n = 1569 tissues and n = 52 cell lines) of the brachyury gene. The prognostic impact of brachyury expression was studied in 1524 glioma patient tumors. The functional impact of brachyury on glioma proliferation, viability, and cell death was evaluated both in vitro and in vivo. Brachyury was expressed in the normal brain, and significantly downregulated in gliomas tissues. Loss of brachyury was associated with tumor aggressiveness and poor survival in glioma patients. Downregulation of brachyury was not associated with gene deletion, promoter methylation, or inactivating point mutations. Brachyury re-expression in glioma cells was found to decrease glioma tumorigenesis by induction of autophagy. These data strongly suggest that brachyury behaves as a tumor suppressor gene in gliomas by modulating autophagy. Importantly, brachyury constitutes an independent positive biomarker of patient prognosis. Our findings indicate that the role of brachyury in tumorigenesis may be tissue-dependent and demands additional investigation to guide rational interventions.
Revista: The Journal of Pathology
Link: https://onlinelibrary.wiley.com/doi/abs/10.1002/path.5419




