Associação Portuguesa de Investigação em Cancro
E-cadherin variants associated with oral facial clefts trigger aberrant cell motility in a REG1A dependent manner
E-cadherin variants associated with oral facial clefts trigger aberrant cell motility in a REG1A dependent manner
The i3S Epithelial Interactions in Cancer group coordinated a multidisciplinary team to address how germline alterations of E-cadherin trigger different clinical manifestations. The group was intrigued by the fact that loss of E-cadherin function may cause hereditary diffuse gastric cancer (HDGC) and congenital malformations, such as orofacial clefts (OFC).
Joana Figueiredo assembled a team of researchers from i3S and University of Coimbra to dissect the mechanisms that are responsible for the abnormal lip/palate closure, the most prevalent birth defects worldwide, with an incidence of 1 in 700 newborns. Affected individuals require comprehensive care as they experience difficulties with feeding, speech, hearing, as well as dental issues and mental health problems.
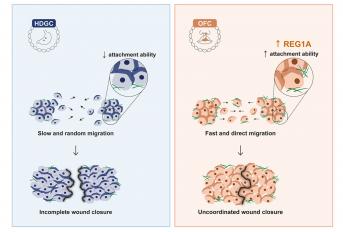
Joana Pereira, a talented PhD student, integrated the project and developed a strategy encompassing in vivo and in vitro models of E-cadherin mutants associated with either OFC or HDGC. A Drosophilaapproach was engineered in collaboration with Morais-de-Sá Lab, demonstrating that variants associated with OFC grant cells an elongated and ergodynamic configuration, which could be linked with less resistance and increased movement across the microenvironment. Through migration assays and time-lapse microscopy, the team confirmed an unusual ability of OFC mutants to migrate faster and with an abnormal directionality, when compared with cells expressing HDGC variants. In fact, although OFC are characterized by failed fusion of the embryonic facial prominences, their results suggest that this may not be the outcome of delayed cell migration. Instead, they propose that an excessive motility performance prevents the coordinated movement of the epithelial cell sheets, generating finger-like projections and irregular leading-edges, which can result in anomalous facial structures. This is supported by a phenotype of mechanical advantage conferred upon improved attachment to extracellular matrices.
The team then hypothesized whether plasticity of OFC mutant cells would be related with the activation of a dedicated expression profile. Following implementation of an RNA-seq workflow, it was verified that the OFC genotype produces a distinct molecular signature from that of HDGC, exposing REG1A as a putative regulator of this outcome.
With this work, the team provided evidence that E-cadherin variants associated with OFC activate aberrant signalling pathways that support dynamic rearrangements of cells towards increased adaptability to the microenvironment. This proficiency results in abnormal tissue shaping and movement, which can be at the heart of orofacial malformations. In the future, genetic counselling of E-cadherin variant carriers could benefit from variant classification pipelines including the assessment of migration features through in vitro assays. This will be crucial to identify those at risk of gastric cancer and those that could have a neonate affected by congenital anomalies.
Ultimately, this manuscript illustrates how a collaborative network with distinct expertise synergized to address a challenging research problem, highlighting the power of site-specific mutant cell lines and fly models in uncovering cellular mechanisms of disease intricacies.
Authors and Affiliations:
Joana Pereira 1 2 3, Soraia Melo 1 2, Rui M Ferreira 1 2, Patrícia Carneiro 1 2, Vítor Yang 1 4 5, André F Maia 1 4, João Carvalho 6, Ceu Figueiredo 1 2 3, José Carlos Machado 1 2 3, Eurico Morais-de-Sá 1 4, Raquel Seruca 1 2 3, Joana Figueiredo 7 8 9
1 i3S - Instituto de Investigação e Inovação em Saúde, Universidade Do Porto, Rua Alfredo Allen, 208, Porto, 4200-135, Portugal.
2 IPATIMUP - Institute of Molecular Pathology and Immunology of Porto University, Porto, Portugal.
3 Faculty of Medicine, University of Porto, Porto, Portugal.
4 IBMC - Institute for Molecular and Cell Biology, University of Porto, Porto, Portugal.
5 ICBAS - Institute of Biomedical Sciences Abel Salazar, University of Porto, Porto, Portugal.
6 CFisUC, Department of Physics, University of Coimbra, Coimbra, Portugal.
7 i3S - Instituto de Investigação e Inovação em Saúde, Universidade Do Porto, Rua Alfredo Allen, 208, Porto, 4200-135, Portugal.
8 IPATIMUP - Institute of Molecular Pathology and Immunology of Porto University, Porto, Portugal.
9 Faculty of Medicine, University of Porto, Porto, Portugal.
Abstract:
Germline mutations of E-cadherin contribute to hereditary diffuse gastric cancer (HDGC) and congenital malformations, such as oral facial clefts (OFC). However, the molecular mechanisms through which E-cadherin loss-of-function triggers distinct clinical outcomes remain unknown. We postulate that E-cadherin-mediated disorders result from abnormal interactions with the extracellular matrix and consequent aberrant intracellular signalling, affecting the coordination of cell migration.
Herein, we developed in vivo and in vitro models of E-cadherin mutants associated with either OFC or HDGC. Using a Drosophila approach, we addressed the impact of the different variants in cell morphology and migration ability. By combining gap closure migration assays and time-lapse microscopy, we further investigated the migration pattern of cells expressing OFC or HDGC variants. The adhesion profile of the variants was evaluated using high-throughput ECM arrays, whereas RNA sequencing technology was explored for identification of genes involved in aberrant cell motility.
We have demonstrated that cells expressing OFC variants exhibit an excessive motility performance and irregular leading edges, which prevent the coordinated movement of the epithelial monolayer. Importantly, we found that OFC variants promote cell adhesion to a wider variety of extracellular matrices than HDGC variants, suggesting higher plasticity in response to different microenvironments. We unveiled a distinct transcriptomic profile in the OFC setting and pinpointed REG1A as a putative regulator of this outcome. Consistent with this, specific RNAi-mediated inhibition of REG1A shifted the migration pattern of OFC expressing cells, leading to slower wound closure with coordinated leading edges.
In conclusion, we provide evidence that E-cadherin variants associated with OFC activate aberrant signalling pathways that support dynamic rearrangements of cells towards improved adaptability to the microenvironment. This proficiency results in abnormal tissue shaping and movement, possibly underlying the development of orofacial malformations.
Journal: Cell Communication and Signaling
Link: https://biosignaling.biomedcentral.com/articles/10.1186/s12964-024-01532-x




